The exploration of hard and super-hard materials has always been an important research direction in the field of condensed matter physics, and it also has great application prospects in practical industrial production. Traditional super-hard materials such as diamond, cubic boron nitride, etc., are usually composed of light elements (BCNO) in the form of covalent bonds. This strong BCNO covalent bond has good directionality and can resist isotropic compression. It also resists shearing in different directions, and therefore exhibits extremely high mechanical strength. However, the defects of traditional superhard materials are also very prominent: diamond is prone to graphitization, and the synthesis of cubic boron nitride requires extremely harsh temperature and pressure conditions. In addition, pure covalent bonding often leads to its electrical insulation or broadband semiconductors. In many applications, industrial applications require materials with superhard mechanical properties and good electrical conductivity (such as superhardness). Coating, wire cutting, press hammer, etc.). Therefore, the search for ultra-hard, low-resistance and even metallic materials has become an important research hotspot in recent years.
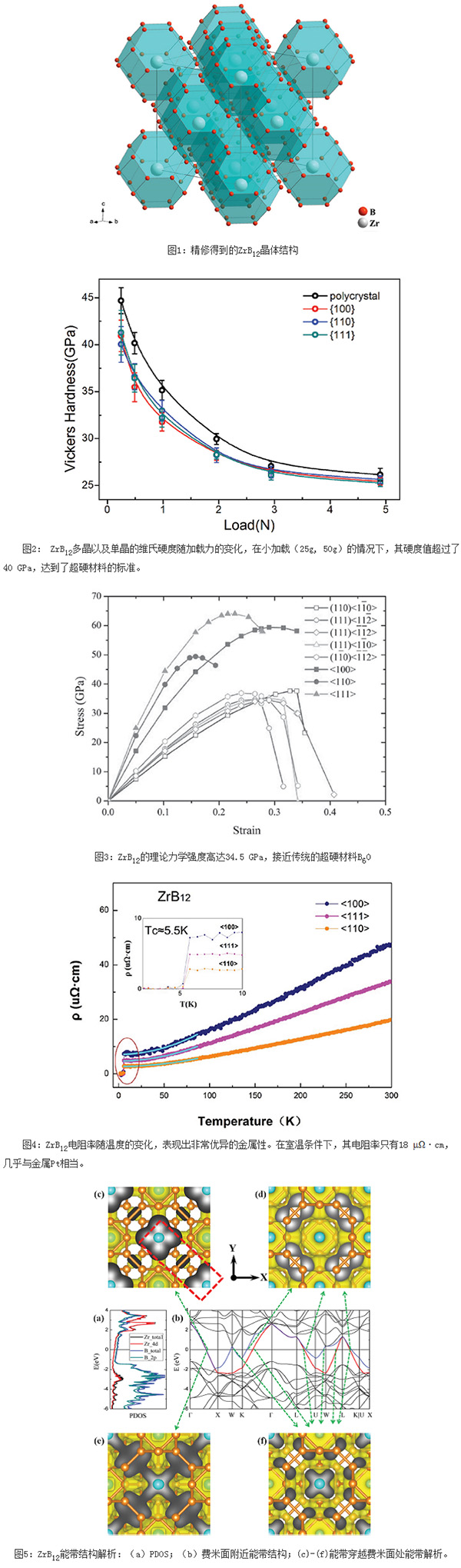
Recently, Institute of Physics, Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics (Extremely) Extreme Conditions Laboratory EX6 Group Research Associate Yu Xiaohui and Surface Physics Laboratory SF10 Group Associate Researcher Li Hui, EX5 Group Researcher Yan Changqing, Jilin University Professor Zhu Pinwen Zhang Ruifeng, a professor of Beijing University of Aeronautics and Astronautics, cooperated and made new progress in the exploration of “metal-electric diamondâ€-ZrB12. They first successfully prepared pure-phase ZrB12 samples by means of high temperature and high pressure, and refined the crystals by crystal diffraction diffraction. As shown in Fig.1: ZrB12 is mainly composed of B network, and the distance between BB is only 1.78 Ã…, corresponding to the extremely strong BB bond, and the crystal is face-centered cubic structure with high symmetry without obvious slip direction. . As shown in Fig. 2, the Vickers hardness in all directions of the crystal is nearly the same, and the mechanical properties are very isotropic. At 50 g loading, ZrB12 has a hardness value of up to 40 GPa, which is the standard for superhard materials; at 500 g loading, the hardness is still as high as 27 GPa, which is 2.5 times more than the most widely used hard WC material at present. . Theoretical calculations show that as shown in Fig. 3, the ideal mechanical strength of ZrB12 can reach 34.5 GPa, which is close to the traditional superhard material B6O formed by pure covalent bonds, and it has good isotropy, which is consistent with the experimental results; The high-symmetry three-dimensional BB network is the intrinsic structural origin of high mechanical properties of ZrB12.
By studying the low-temperature electrical transport properties of ZrB12 single crystals, they found that ZrB12 has very excellent metal properties. As shown in Figure 3, at room temperature, the resistivity is only 18 μΩ·cm, which is almost equal to that of metal Pt; with the decrease of temperature, the resistivity change of ZrB12 also shows a metallic behavior, and around 5.5 K. Superconductivity has appeared. In addition, the seeback coefficient of ZrB12 at room temperature is only 2.0 μV·K-1, which also indicates that this material has good metal properties. Based on the structural data obtained by crystal diffraction refinement, they found that the structural body of ZrB12 is formed by the BB three-dimensional cage network, while the transition metal Zr is in the center of the {B}28 cage, with a distance of 5.2 from the adjacent Zr. Å, which means that there will be no direct metal orbital overlap; and the BB network shows exceptionally good mechanical stability indicating that the BB bond is a local covalent form. To understand the superior metallic properties of ZrB12 beyond the ultra-hard properties, the researchers took a first-principles calculation simulation. They found that Zr atoms, when bonded to B, provide a large amount of electrons to the B orbit. On average, each Zr atom transfers 2.6 electrons to the Zr-B hybrid orbital. As shown in Fig. 5, further analysis of the ZrB12 band structure reveals that the Zr-B hybrid orbitals can be superimposed on the BB three-dimensional orbitals to form a d-π-d bridge structure and the entire crystal structure. The formation of a continuous delocalized conductive path makes the ZrB12 as a whole exhibit exceptionally superior metallicity. It can be said that the BB 3D network is not only an important support for the crystal structure of ZrB12, but also a bridge for rapid electron conduction.
The related results were recently published in Advanced Materials (DOI: 10.1002/adma.201604003). The research work was funded by the National Natural Science Foundation of China.
Material :Natural slate , quartz,sandstone ,marble etc .
Size :1-5mm,5-10mm ,4-8mm,8-12mm ,12-18mm etc .Can make the shape as your requirement
Shape :machine process :gravel or pebble stones
Packing : wooden pallets.
The wooden pallets size is made as the container size . After loading the wooden crate in the container ,the wooden crates will nearly same size with the width of the container .It can make the wooden crate not have space to move during transport . In this case ,it can keep the stone safety mostly
Application : Can be used to decorate the outside wall or inside wall .Decorate your house ,decorate your life .
Gravel Pebble,Decorative Stones,Gravel Pebble Stone,Garden Pebbles Gravel
HEBEI DFL STONE , https://www.dflstone.com